1. Konec produktuview
The Healgen Rapid Check COVID-19, Flu A&B Antigen Test Kit is an over-the-counter (OTC) diagnostic device designed for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2, influenza A, and influenza B in direct anterior nasal swab specimens. This test is intended for use by individuals aged 2 years and older, providing results in approximately 15 minutes. It features two distinct result windows for clear differentiation between COVID-19 and Flu A/B.
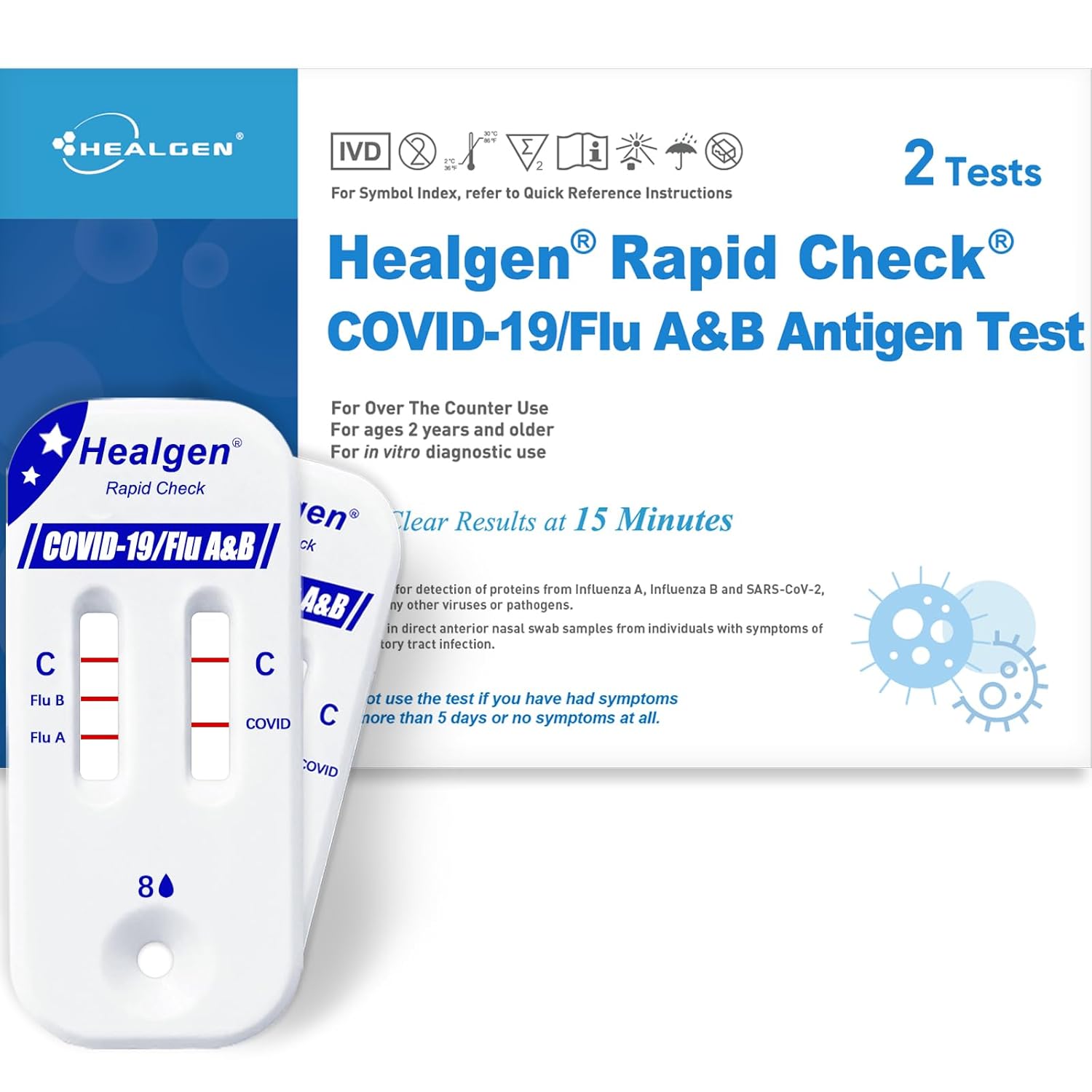
Image: The Healgen Rapid Check COVID-19, Flu A&B Antigen Test Kit, showing the box and the test cassette with dual windows.
2. Obsah sady
Each Healgen Rapid Check COVID-19, Flu A&B Antigen Test Kit contains the following components:
- Swab: For specimen collection.
- Tube: Pre-filled with extraction buffer.
- Cassette: The test device with two result windows.
- QRI: Quick Reference Instructions.
3. Důležité bezpečnostní informace
- Pro in vitro diagnostic use only. Do not ingest any components of the kit.
- Keep out of reach of children. Adult assistance is required for individuals aged 2-13 years during sample collection. Individuals aged 14 years and older can self-collect.
- Do not use the test if the packaging is damaged or if the expiration date has passed.
- Follow all instructions carefully to ensure accurate results. Incorrect sample collection or test procedure may lead to inaccurate results.
- Dispose of used test components according to local regulations for biohazardous waste.
- Consult a healthcare professional if you have questions about your test results or if your symptoms worsen.
4. Nastavení a příprava
Before beginning the test, ensure you have a clean, flat surface and all kit components are at room temperature. Wash your hands thoroughly with soap and water or use an alcohol-based hand sanitizer before handling the test kit.
- Open the kit box and remove all contents.
- Check the expiration date on the foil pouch of the test cassette. Do not use if expired.
- Tear open the foil pouch and remove the test cassette. Place it on a clean, flat surface.
- Ensure the pre-filled extraction tube is securely capped until ready for use.
5. Návod k obsluze
Follow these steps carefully to perform the test:
- Krok 1: Odběr tamponu
Carefully insert the entire soft tip of the swab into one nostril about 1/2 to 3/4 inch (1 to 1.5 cm). Firmly rub the swab in a circular motion against the inside wall of the nostril at least 5 times for 15 seconds. Using the same swab, repeat the process in the other nostril. Ensure both nostrils are adequately sampvedený.

Image: Diagram illustrating the correct method for nasal swab collection, showing circular motions in both nostrils.
- Step 2: Mix the Sample
Unscrew the cap from the pre-filled extraction tube. Insert the swab tip into the tube, ensuring it reaches the bottom. Swirl the swab vigorously in the liquid at least 6 times, pressing the tip against the bottom and sides of the tube to release the sample. Remove the swab while squeezing the sides of the tube to extract as much liquid as possible from the swab.
- Step 3: Drop onto Cassette
Replace the cap securely onto the extraction tube. Invert the tube and dispense exactly 8 drops of the solution into the sample well(s) of the test cassette. The test cassette has two separate wells, one for Flu A/B and one for COVID-19. Ensure drops are dispensed into both wells.
- Step 4: Wait for Result
Set a timer for 15 minutes. Do not read the results before 15 minutes or after 20 minutes, as this may lead to inaccurate readings.
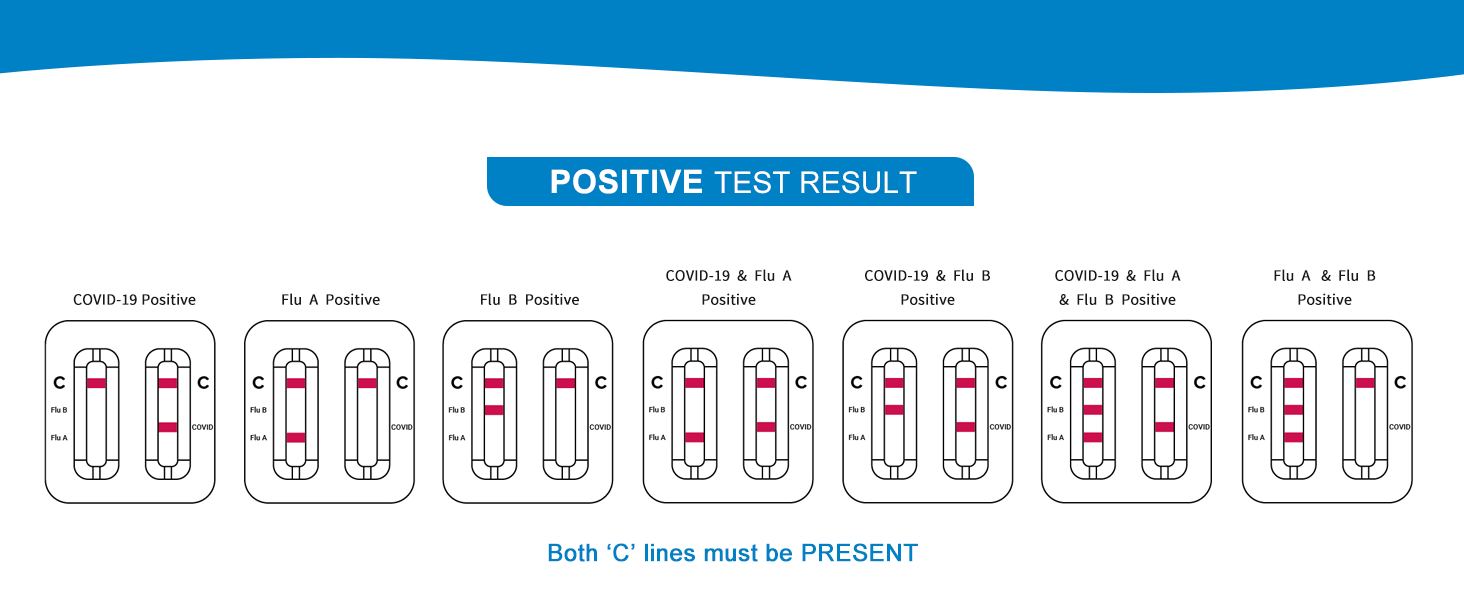
Image: A visual guide to the four simple steps of the test: Swab, Mix, Drop, and Wait for 15 minutes.
6. Interpretace výsledků
Results should be read at 15 minutes. The test cassette has two distinct result windows: one for Flu A/B and one for COVID-19. Each window will display a Control line (C) and a Test line (T) if present.
Pozitivní výsledek
A positive result is indicated by the presence of obě the Control line (C) and a Test line (T) in the respective window (Flu A/B or COVID-19). Even a faint test line should be considered positive.
- COVID-19 Positive: Control line (C) and Test line (T) appear in the COVID-19 window.
- Flu A Positive: Control line (C) and Test line (T) appear in the Flu A/B window, with the T line aligning with 'Flu A'.
- Flu B Positive: Control line (C) and Test line (T) appear in the Flu A/B window, with the T line aligning with 'Flu B'.
- Co-infection: Positive results may appear in both windows, or both Flu A and Flu B lines may appear in the Flu A/B window.
Obrázek: Vizuální examples of various positive test results, including COVID-19, Flu A, Flu B, and combinations thereof.
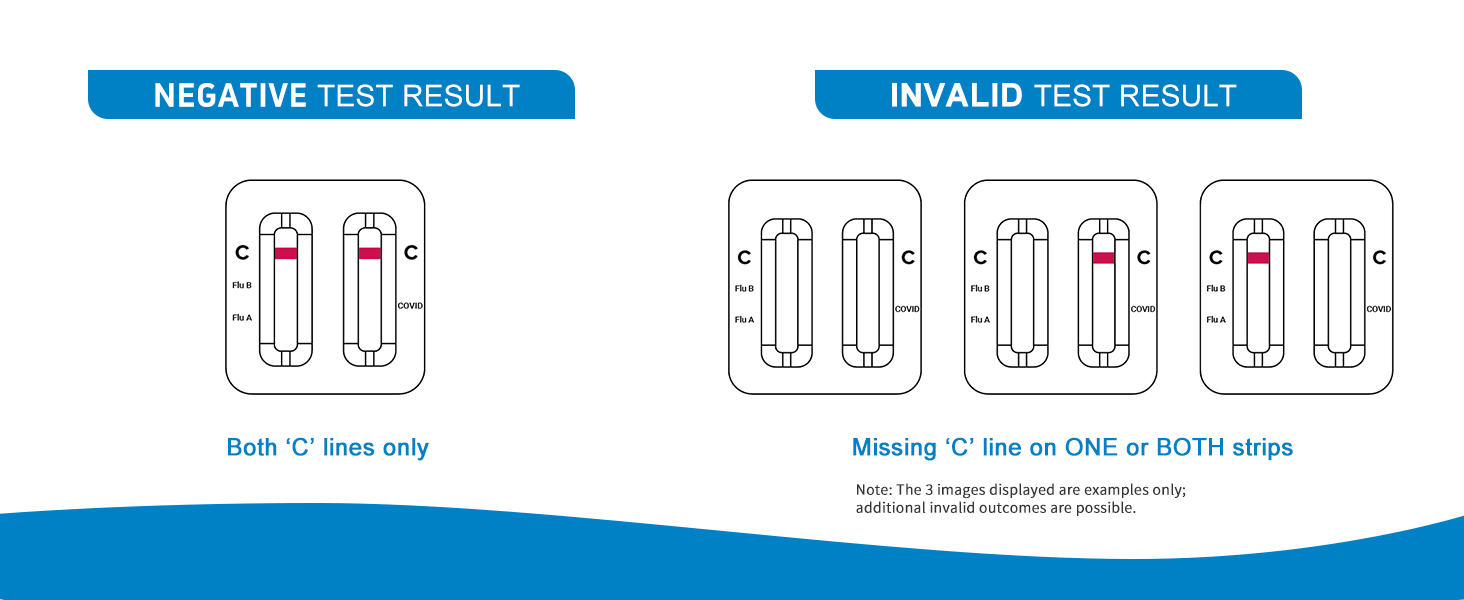
Negativní výsledek
A negative result is indicated by the presence of only the Control line (C) in both the Flu A/B and COVID-19 windows. No Test line (T) should be visible in either window.
Neplatný výsledek
An invalid result occurs if no Control line (C) appears in either or both windows, regardless of whether a Test line (T) is present. An invalid result means the test did not work correctly. In this case, a new test should be performed with a new kit.

Obrázek: Vizuální examples of a negative test result (only control lines) and invalid test results (missing control lines).
Důležitá poznámka: The images displayed are examples only; additional outcomes, including co-infection, are possible. Always refer to the Quick Reference Instructions (QRI) included in your kit for the most detailed interpretation guide.
7. Skladování a údržba
The Healgen Rapid Check COVID-19, Flu A&B Antigen Test Kit is designed for single use and requires minimal maintenance. Proper storage is crucial to maintain the integrity and effectiveness of the test components.
- Store the test kit at room temperature, between 2°C and 30°C (36°F and 86°F), in its original sealed packaging.
- Nezmrazujte soupravu.
- Keep the test cassette sealed in its foil pouch until immediately before use to protect it from moisture.
- The kit has an 18-month shelf life from the date of manufacture. Always check the expiration date printed on the packaging before use.

Image: Information regarding the FDA extended shelf life for Healgen test kits, indicating various lot numbers and their original and extended expiration dates.
8. Řešení problémů
If you encounter issues during testing, refer to the following common problems and solutions:
| Problém | Možná příčina | Řešení |
|---|---|---|
| No lines appear (Control or Test). | Invalid test. Insufficient sample volume, incorrect procedure, or damaged test cassette. | Repeat the test with a new kit, ensuring all steps are followed precisely, especially adding the correct number of drops. |
| Only a Test line (T) appears, no Control line (C). | Invalid test. The test did not run correctly. | Repeat the test with a new kit. The Control line must always be present for a valid result. |
| Faint Test line (T) appears. | This is still considered a positive result. | Any visible line, no matter how faint, in the Test region along with a Control line, indicates a positive result. |
| Výsledky jsou nejasné nebo rozmazané. | Příliš mnoho nebo příliš málo sample, or reading outside the 15-20 minute window. | Ensure exactly 8 drops are added. Read results strictly within the 15-minute timeframe. If still unclear, repeat with a new kit. |
If problems persist or you have concerns about your results, contact a healthcare professional or the manufacturer's customer support.
9. Specifikace produktu
- Název produktu: Healgen Rapid Check COVID-19, Flu A&B Antigen Test Kit
- Číslo modelu: B0DKXY315G
- Cíl detekce: SARS-CoV-2 nucleocapsid protein antigen, Influenza A nucleoprotein, Influenza B nucleoprotein
- Sample Typ: Přední výtěr z nosu
- Čas zkoušky: 15 minut
- Doba použitelnosti: 18 měsíců
- Skladovací teplota: 2 °C - 30 °C (36 °F - 86 °F)
- Výrobce: Healgen Scientific, LLC.
- Rozměry balení: 4.96 x 3.19 x 1.22 palce
- Hmotnost balení: 1.45 unce
10. Záruka a podpora
For specific warranty information regarding the Healgen Rapid Check COVID-19, Flu A&B Antigen Test Kit, please refer to the documentation included with your purchase or contact Healgen Scientific, LLC. directly. The product has an 18-month shelf life, which indicates its usability period, not a warranty against defects.
For technical support or inquiries, please visit the official HEALGEN website or contact their customer service department. Contact information is typically provided on the product packaging or the manufacturer's webmísto.
11. Instruktážní videa
No official instructional videos from the seller were provided for embedding at this time. Please refer to the written instructions and diagrams provided in this manual and the Quick Reference Instructions included in your kit for guidance on test procedures.